Beyond its ability to degrade tau amyloid fibrils, the serine protease HTRA1 displays an unprecedented dual ability to both disaggregate tight amyloid cores as well as cleave the newly-extricated polypeptides.
(À la Nature News & Views style, i.e. a more technical tone)
Misfolded proteins and insoluble protein aggregates can have devastating consequences and amyloid fibrils are associated with diseases such as Alzheimer’s, Parkinson’s, and Huntington’s1. Evolution has provided cells with a small arsenal of chaperones, cochaperones, and proteases to disentangle protein aggregates and to deliver the more manageable soluble polypeptides for refolding or proteolysis. Nevertheless, after decades of research, the full scope of these responses is still not fully understood. Of what is known, these protective proteins typically play a single role in the process: they disentangle, or they digest, or they refold. Amongst the classical suite of heat shock proteins, the coordination of three proteins (Hsp 40, 70, and 104) is needed simply to disaggregate insoluble protein2. In this context, the high-temperature requirement A1 (HTRA1) serine protease, is an intriguing anomaly. While already noteworthy for its ability to degrade tau amyloid fibrils3, the recent investigation by Poepsel et al. shows that HTRA1 has a novel dual capacity to both unravel fibrils and then to directly digest the newly vulnerable amyloid strands4.
The HtrA family is a highly conserved group of ATP-independent serine proteases acting as protein quality control factors with tightly regulated individual roles including the detection, repair, and degradation of misfolded proteins4. Human HTRA1 has both extra- and intracellular activities; however, its association with microtubules and its ability to degrade tubulin triggered further studies on HTRA1’s potential to disaggregate amyloid fibrils5. Tau, another microtubule-associated protein, is tame and functional in its soluble form, but notoriously prone to aggregation whereby the resulting fibrils are harbingers of taupathies such as Alzheimer’s Disease6. Due to tau’s clinical relevance and the probable proximity of tau and HTRA1 on microtubules, Ehrmann’s group speculated that HTRA1 could also be capable of degrading tau fibrils. Their investigation showed that HTRA1 not only disrupted tau fibrils, but that HTRA1 mRNA and activity were upregulated in response to increased tau and, even more intriguingly, HTRA1 and tau levels appeared to be inversely correlated in the brains of Alzheimer’s patients3.
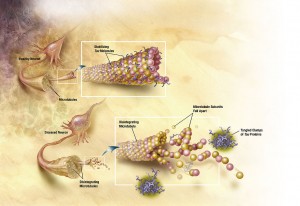
These findings prompted the current investigation on the mechanistic details of how HTRA1 degrades tau fibrils, in which Poepsel et al. performed a battery of experiments dissecting this novel biological approach towards removing amyloid fibrils4. To tease apart the mechanics of HTRA1 operation, Poepsel et al. began by creating the proteolytically inactive version HTRA1S328A, designed to isolate HTRA1’s disaggregating activity from its proteolytic function. In addition to the serine protease domain, HTRA1 is composed of a signal sequence for secretion, an incomplete insulin-like growth factor binding protein (IGFBP-7) domain, and a C-terminal PDZ domain, which is thought to play a role in extracting tau monomers. Regardless of its incapacitated protease domain, HTRA1S328A maintained its ability to disaggregate tau fibrils both in cell-free and cellular models (additionally confirmed and quantified on a single-molecule level with atomic force microscopy). In line with reasoning that disaggregation primes misfolded proteins for future processing, the next question was whether disaggregation by HTRA1S328A increased the efficiency of subsequent proteolysis by native HTRA1. Another collection of experiments showed that HTRA1 proteolysis with significantly more efficient when fibrils were first preincubated with HTRA1S328A. Fibril degradation was nearly tripled when samples were preincubated with HTRA1S328A prior to proteolysis with HTRA1 (as quantified by atomic force microscopy).
Another remaining question was whether HTRA1’s activity was restricted to the ends of fibrils or whether it could dismantle at any point along the fibril. By visualizing the fibrils with electron microscopy, the authors observed association of HTRA1S328A along the length of the fibrils, which they also confirmed with real-time reductions of fluorescence showing uniform degradation across the fibrils. The specificity of tau proteolysis was furthermore confirmed with mass spectrometry and isothermal titration calorimetry and, consistent with prior findings, alteration of the PDZ domain disrupted HTRA1’s disaggregation activities.
While other HtrA family members have shown dual disaggregating and refolding activity, this is the first appearance of disaggregation and proteolysis within the same protein. In addition, throughout these investigations, soluble tau was curiously impervious to the proteolytic activity of HTRA1, despite up to 10-fold increases in HTRA1. The underlying mechanisms of how HTRA1 distinguishes between tau forms will be the subject of future investigations, as well as the roles of HTRA1 in human disease. Beyond Alzheimer’s disease, substrates or products of HTRA1 have also been implicated in age-related macular degeneration, arthritis, familial ischemic cerebral small-vessel disease, and some cancers and a more thorough understanding of HTRA1 may eventually aid the prevention or treatment of such diseases7-11.
References
1. Friedman, R. Aggregation of amyloids in a cellular context: modelling and experiment. Biochem. J. 438, 415–426 (2011).
2. Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14, 630–642 (2013).
3. Tennstaedt, A. et al. Human high temperature requirement serine protease A1 (HTRA1) degrades tau protein aggregates. J. Biol. Chem. 287, 20931–20941 (2012).
4. Poepsel, S. et al. Nat Chem Biol. 2015 Oct 5. [Epub ahead of print]
5. Chien, J. et al. Serine protease HtrA1 associates with microtubules and inhibits cell migration. Mol. Cell. Biol. 29, 4177–4187 (2009).
6. Spillantini, M.G. & Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622 (2013).
7. Chien, J., Campioni, M., Shridhar, V. & Baldi, A. HtrA serine proteases as potential therapeutic targets in cancer. Curr. Cancer Drug Targets 9, 451–468 (2009).
8. Yang, Z. et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 314, 992–993 (2006).
9. Grau, S. et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA 102, 6021–6026 (2005).
10. Milner, J.M., Patel, A. & Rowan, A.D. Emerging roles of serine proteinases in tissue turnover in arthritis. Arthritis Rheum. 58, 3644–3656 (2008).
11. Hara, K. et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N. Engl. J. Med. 360, 1729–1739 (2009).
Image credits:
By GerryShaw (Own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons
By ADEAR: “Alzheimer’s Disease Education and Referral Center, a service of the National Institute on Aging.” [Public domain], via Wikimedia Commons

